The challenge – Early diagnosis of bacterial infections in critically ill patients
Early diagnosis of bacterial infections in critically ill patients is challenging, as the clinical manifestation is non-specific. Furthermore, the results from microbial cultures may be delayed, resulting in delayed antibiotic treatment, which is associated with increased mortality. Neutrophil activation is an early and major response to bacterial infections. Calprotectin constitutes 40-60% of cytosolic protein content in neutrophils and is an early marker for neutrophil activation.
Rapid release of calprotectin
Several studies have shown rapid release of calprotectin upon stimulation with endotoxin and/or E.Coli 1,2 and the ability of calprotectin to predict bacterial infections before onset of clinical symptoms3. With early diagnosis of bacterial infections delayed treatment will be avoided as well as deterioration due to severe infection/sepsis4.
Early detection of infection at the ICU
A health economic model is employed to estimate the cost-effectiveness of analysis of calprotectin for early detection of bacterial infection and thus, the earlier start of antibiotic treatment compared to other diagnostic biomarkers. The model has been developed, based on the results from a previous study showing ability of calprotectin to detect bacterial infections before onset of clinical symptoms and antibiotic prescription3.
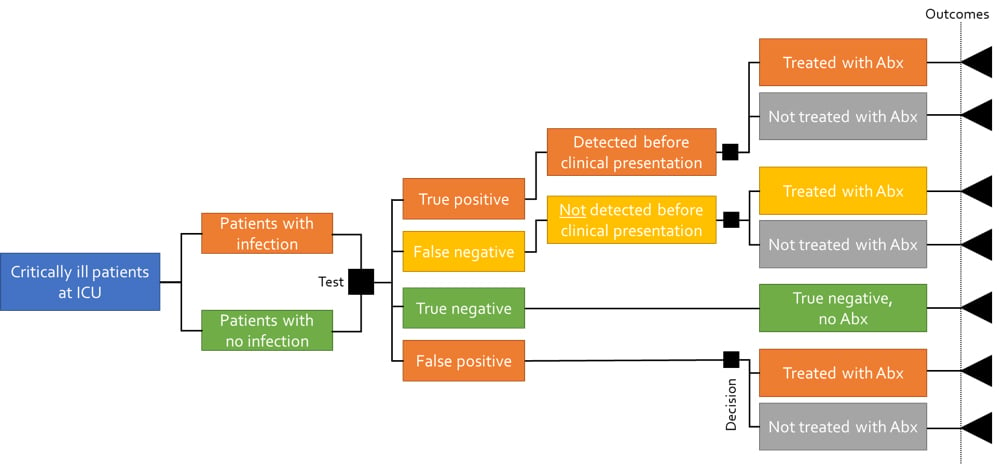
Figure 1. Decision-tree structure with a predictive test
The analysis is based on patients admitted to an intensive care unit (ICU) in Sweden, with inclusion and exclusion criteria from literature applied. The model allows for different diagnostic outcomes based on correctly and incorrectly diagnosis of bacterial infection and timing of antibiotic treatment:
- Patient survival
- Length of stay in ICU and in general ward
- Total costs of treatment during hospital stay.
The comparators included in the analysis are:
- White blood cell count (WBC)
- Procalcitonin (PCT)
- C-reactive protein (CRP)
- No testing
Reduced cost, mortality rate and mean length of stay
In the base-case scenario, analysis of calprotectin is employed predictively, differently to the comparators that are employed diagnostically. This means that the proportion of calprotectin tests taken predictively is 100%, while the comparators are set to 0%.
The base-case results show that predictively measuring of calprotectin in an ICU setting, using the GCAL® calprotectin assay reduces total costs by approximately 13 000 – 18 000 EUR per patient, overall mortality rate by 0.11, and mean length of stay in an ICU and general ward by 1.3 – 2 days and 6.9 – 8 days, respectively.
|
Δ Total cost (EUR) |
Δ Deaths |
Δ Mean days ICU |
Δ Mean days ward |
|
|
Calprotectin |
Reference |
Reference |
Reference |
Reference |
|
White blood cell count |
+12 683 |
+0.11 |
+1.27 |
+7.12 |
|
Procalcitonin |
+13 756 |
+0.11 |
+1.42 |
+7.27 |
|
C-reactive protein |
+13 700 |
+0.11 |
+1.46 |
+6.88 |
|
No test |
+17 522 |
+0.11 |
+1.97 |
+7.82 |
Table 1. Base-case outcomes (difference compared to calprotectin) per comparator/per patient
Sensitivity analysis
Sensitivity analysis was applied to various uncertain parameters in the model. These parameters included:
- The proportion of predictive tests for comparators where comparators were used predictively in 50% of patients
- Variation of length of stay in ICU
- General ward and in-patient costs were cost of in-patient care per day were reduced by 20%.
In all sensitivity analyses, calprotectin remains the dominant option when key model inputs are varied.
Conclusion
The base-case scenario in the presented model identified calprotectin as cost-effective biomarker for a patient cohort presented in a Swedish ICU. Compared to the comparators, PCT, CRP and WBCs calprotectin, analysed by the GCAL® Calprotectin Immunoassay was shown to save total costs, reduce the mean duration of in-patient care, and reduce in-hospital mortality in those patients. Although this study focuses on a health economic perspective, the main rationale of analysis of calprotectin is from a clinical perspective, since early diagnosis of severe infections and sepsis reduces both delays in treatment and mortality. From this aspect, data from the health economic model support previous studies, where early detection of severe infections and sepsis has both cost-saving and life-saving impact in the ICU setting.
Contact us for further information about calprotectin and the GCAL® assay
Explore GCAL® in your clinical practice and laboratory - fill out the form or send an email to marketing@gentian.com for more information about the product and prices.
References:
- Fullerton J et al. Kinetics of calprotectin, procalcitonin and C-reactive protein in healthy volunteers administered intravenous endotoxin. 40th International Symposium on Intensive Care & Emergency Medicine. Critical Care. 2020. P474
- Lipcsey M et al. The time course of calprotectin liberation from human neutrophil granulocytes after Escherichia coli and endotoxin challenge. Innate Immun. 2019;25(6):369-373
- Jonsson N et al. Calprotectin as an early biomarker of bacterial infections in critically ill patients: an exploratory cohort assessment. Crit Care Resusc. 2017;19(3):205-213
- Ferrer R et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749-1755
*have been presented at the International Symposium on Intensive Care and Emergency Medicine (ISICEM) meeting in Brussels.
Authors: A Havelka1 M Lipcsey2 M Hultström2 A Larsson3
1) Gentian Diagnostics, Stockholm, Sweden, 2) Uppsala University, Department of Surgical Sciences, Anesthesiology, Uppsala, Sweden, 3) Akademiska University Hospital, Department of Medical Sciences, Clinical Chemistry, Uppsala, Sweden


-1.jpg)
.png)