
Introduction: Gentian SARS-CoV-2 Total Antibody assay *
The outbreak of the SARS-CoV-2 pandemic and continuous presence of the virus in the community require efficient tests for monitoring the anti-SARS-CoV-2 levels and management of the vaccination response in the population.
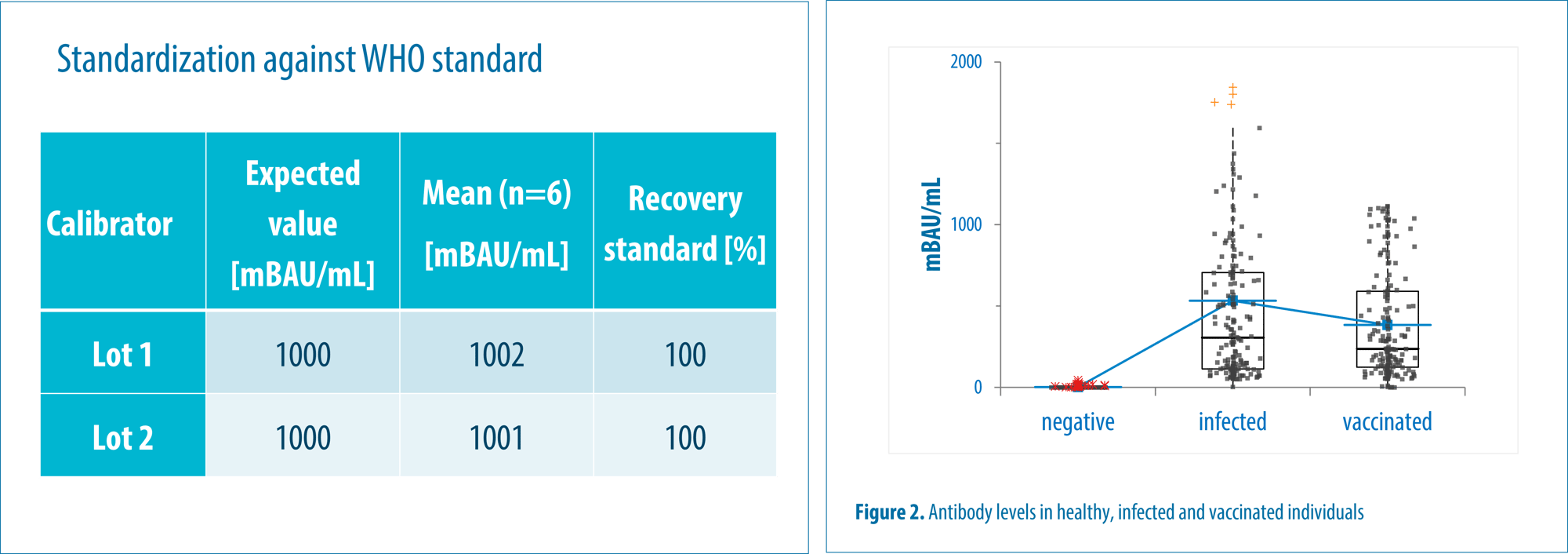
Gentian AS, in collaboration with the University of Tromsø, has developed a SARS-CoV-2 Total Antibody assay. The test is adapted to WHO's international standard and enables comparison of results across laboratories and other antibody tests. The Gentian SARS-CoV-2 Total Antibody assay can be used on several turbidimetric instruments regardless of the manufacturer. The test is fully automated, which provides increased test capacity and efficiency in the laboratory with short time to results.
Materials and Methods
The Gentian SARS-CoV-2 assay was developed and validated on the turbidimetric instruments Cobas c501 from Roche and BS240 BS/240Pro from Mindray. Samples from healthy donors or patients, collected before COVID-19 pandemic were used for evaluation of clinical specificity. Samples from patients infected with the SARS-CoV-2 virus or vaccinated against the virus were used for evaluation of clinical sensitivity.
Results
The assay has a calibration range from 0 to 1000 mBAU/mL. No antigen excess was observed up to 2500 BAU/mL. Precision achieved a CV <20 % for samples with a concentration ≤ 165 BAU/mL, and <10 % for samples >165 BAU/mL.
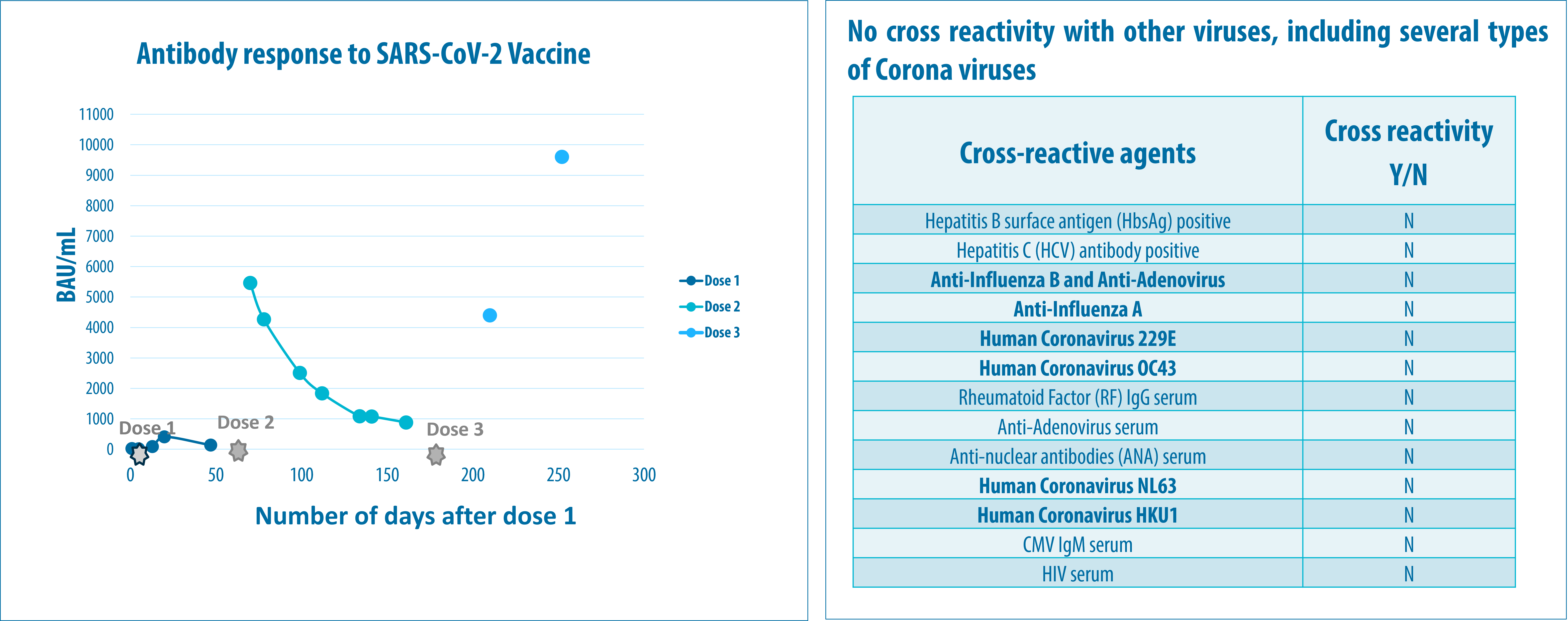
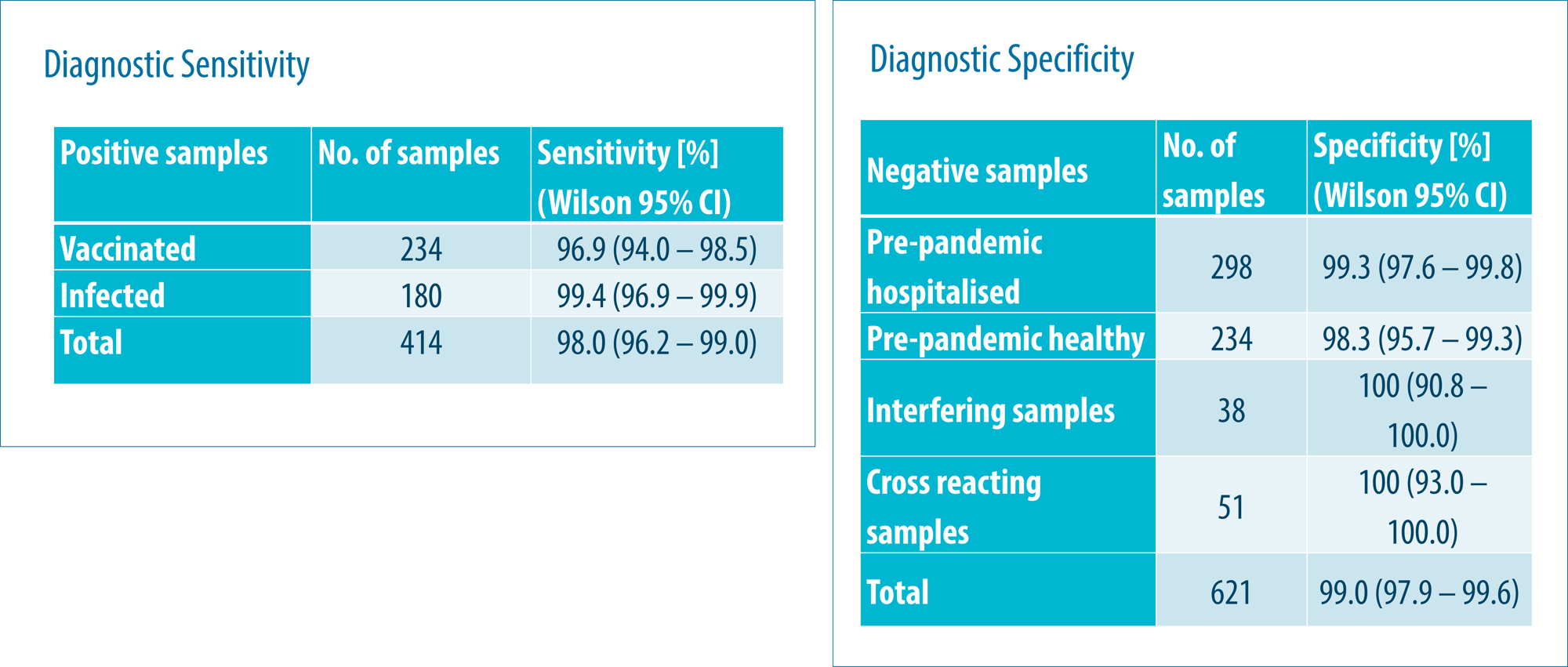
No clinically relevant cross reactivity towards 13 antibodies, including antibodies against other Corona viruses was observed. Serum from patients infected with the Alpha, Beta, Gamma, Delta and Omicron, variants were all positive. The assay was able to detect antibodies generated in response to different vaccines and Alpha, Beta, Gamma, Delta and Omicron (BA 1, BA 3, and BA5) virus variants. Sensitivity and specificity of the assay met the acceptance criteria set out from the MDCG 2021-21 guideline, ≥90% and >99% respectively.
Conclusion
The Gentian SARS-CoV-2 Total Antibody assay can be a powerful tool for the long-term monitoring and community management of COVID-19. It is a reliable high-throughput assay with high sensitivity and specificity that assess antibody response after a natural infection or vaccination.
The assay can be used to monitor immunity in the population, ensure effective and reliable monitoring of the virus infections and help with long-term monitoring, planning and management of the vaccination response in the community.
Contact us for more information
Do you want more information about the assay or want to validate the assay in your laboratory?
Please send us an email to marketing@gentian.com or fill out the form below. Our Product Manager for SARS-CoV-2 Total Antibody Immunoassay will get in touch.
Poster presented at the Swedish Clinical Chemistry meeting in Jönköping 2022
*This article is based on a poster presented at the Swedish Clinical Chemistry meeting in Jönköping 2022
Authors
Havelka Aleksandra1,2, Nilsen Tom1, Safari Asad1, Haukebø Anette1, Trosten Ella1, Arnlund David3 and Olsvik Ørjan4
- Gentian AS, Moss, Norway
- Department of Molecular Medicine and Surgery, Karolinska Institute, Stockholm, Sweden
- Getica AB, Gothenburg, Sweden
- Department of Medical Microbiology, Faculty of Medicine, UiT Norwegian Arctic University, Tromsø, Norway



